
Novel Therapies for Mesothelioma
Several new agents have recently been approved or are soon to be approved by the FDA for the treatment of cancer. [1] These agents are very interesting compounds, and further research is needed to determine their precise role in the treatment of cancer. The basic properties of these agents are outlined in Table 1.
Table 1. New Anticancer Agents*
View Table 1. New Anticancer Agents
| Drug | Classification | Indication | Dose and Administration | Common Toxicities |
| Temozolamide (Temodar) | Alkylating agent | Refractory anaplastic astrocytoma that has failed prior chemotherapy | 150 mg/m 2/day orally for 5 days. Cycle is to be repeated every 28 days depending on count recovery. Medication should be taken on an empty stomach. Dose should be adjusted to keep ANC = 1000-1500/mm 3 and platelets = 50,000-100,000/mm 3 | Myelosuppression (white blood cells and platelets) Nadir occurs at 28 days. Cycle should not be repeated unless counts are adequate. Nausea and vomiting--may be decreased if dose administered at bedtime. Headache, fatigue, constipation |
| UFT and Leucovorin (Orzel) | UFT: Antimetabolite activityLeucovorin: Potentiates activity of 5-fluorouracil (5-FU) | Metastatic colorectal cancer | UFT 300 mg/m 2/day orally X 28 days with 1-week rest period. Leucovorin: 75-90 mg/day in 3 divided doses | Myelosuppression, diarrhea, mucositis, fatigue, increased bilirubin. Does NOT cause hand-foot syndrome seen with 5-FU and its analogues. |
| Epirubicin (Ellence) | Anthracycline analogue | Adjuvant therapy in node-positive breast cancer in patients who have undergone surgery | 100-120 mg/m 2 as a single IV infusion or in 2 divided doses on days 1 and 8 of each cycle of therapy | Myelosuppression, nausea and vomiting, alopecia, mucositis, vesicant properties, secondary malignancies. Maximum recommended cumulative dose = 900 mg/m 2 |
| Valrubicin (Valstar) | Anthracycline analogue | Bladder cancer (insitu) that is refractory to BCG therapy in patients who have contraindications for immediate cystectomy | 800 mg intravesicularly weekly; dose should be diluted to 75 ml and retained for 2 hours. Valrubicin is in Cremophor EL base, so non-DEHP containers and administration sets should be used. | Urinary frequency, dysuria, urinary urgency. Systemic toxicities of anthracyclines (myelosuppression, mucositis, nausea/vomiting) are seen in patients with non-intact bladders. |
| Denileukin diftitox (Ontak) | Fusion protein | Persistent or recurrent cutaneous T-cell lymphoma expressing the CD25 component of the IL-2 receptor | 9-18 mcg/kg/day IV over 15 minutes daily X 5 days. Cycle is repeated every 3 weeks. Product must be stored in the freezer. Product must be diluted with NS. Glass containers should be avoided and the product should not be filtered. Must use prepared solution within 6 hours of preparation. | Acute hypersensitivity reactions, capillary leak syndrome (hypotension, hypoalbuminemia, edema), chills, fever, asthenia, headache, nausea/vomiting, anorexia, diarrhea, infection, pain at tumor site, pruritus, increased transaminases. |
* Some of these agents have yet to be approved by the FDA.
Temozolamide
Temozolamide is an oral alkylating agent that is similar to dacarbazine. Temozolamide undergoes spontaneous hydrolysis to MTIC, which is further metabolized to 5-aminoimidazole-4 carboxamide (AIC) -- the active cytotoxic compound. Because of the spontaneous hydrolysis of the compound, temozolamide has less variability in its conversion to MTIC than does dacarbazine, and therefore there are differences in activity, spectrum, and potency between the 2 agents. Temozolamide is almost 100% bioavailable. There is decreased absorption with food, so it is recommended that the doses be taken on an empty stomach. Temozolamide crosses the blood-brain barrier - hence, its activity in primary brain tumors and brain metastasis. Delayed myelosuppression is the dose-limiting toxicity. Nausea and vomiting may be less severe if doses are taken on an empty stomach at bedtime. Potential future uses for temozolamide include the treatment of glioblastomas, melanoma, and sarcoma. The cost of 1 course of therapy is approximately $1560 (average wholesale price [AWP]).
UFT
UFT is a combination product that contains uracil and ftorafur in a 4:1 ratio. Ftorafur is a 5-fluorouracil (5-FU) prodrug and is combined with uracil, which inhibits the metabolism of 5-FU. Leucovorin is added to the UFT therapy as a potentiation agent. Leucovorin increases the binding of 5-FU to thymidalate synthase, thereby increasing the cytotoxicity of 5-FU. Ftorafur is well absorbed orally and slowly metabolized. Surprisingly, UFT + leucovorin does not cause hand-foot syndrome, which is a dose-limiting toxicity of 5-FU therapy and the other available 5-FU prodrugs (ie, capecitabine). Future uses for this combination may be for the treatment of gastrointestinal malignancies, breast cancer, and head and neck tumors. The relative cost of UFT/leucovorin is unknown; however, when evaluating the cost of the regimen, it is important to keep in mind that 5-FU therapy requires IV administration involving pumps and preparation time. Also, the average cost for a course of capecitabine is approximately $770 (AWP).
Epirubicin
Epirubicin is an anthracycline analogue that has been used in Europe for several years. It is believed that epirubicin may have a lower incidence of cardiotoxicity; however, there is little evidence to support this. Epirubicin has a large volume of distribution and undergoes extensive hepatic and biliary excretion. Dose modifications are required for patients with hepatic dysfunction. There is evidence that there is decreased clearance of epirubicin in elderly females. Cimetidine increases the area under the curve of epirubicin by 50% and decreases the clearance of the drug by 30%. Future uses of epirubicin may include treatment of metastatic breast cancer and treatment of other solid tumors sensitive to anthracycline therapy.
Valrubicin
Valrubicin, a semisynthetic analogue of doxorubicin, is a highly lipophilic compound solubilized in Cremophor EL. It is hypothesized that because of decreased interaction with negatively charged molecules, valrubicin may be less irritating to local tissue than other agents used for intravesicular administration. Valrubicin penetrates the bladder tissue with negligible absorption and metabolism in patients with an intact bladder. If patients have perforated bladders or inflamed bladders, there is risk of systemic toxicities from valrubicin. Clinical trials showed that valrubicin therapy in patients who had failed bacillus Calmette-Guérin (BCG) therapy le ad to more durable responses; however, there was a high incidence of metastatic disease in patients treated with valrubicin. This increase in metastatic disease may be attributable to the fact that cystectomy was delayed in patients receiving valrubicin. Therefore, it is recommended that therapy be discontinued in patients not responding after 3 months of treatment. The cost of therapy is substantial at $9360 for 6 weeks of treatment. Future possibilities for valrubicin may be in the front-line treatment of carcinoma insitu of the bladder.
Denileukin diftitox
Denileukin diftitox is a fusion protein that directs diphtheria toxin to cells that express the CD25 component of the IL-2 receptor. It is interesting that all patients who receive denileukin will develop antibodies against the protein. There is also an increase in the clearance of the drug with prolonged exposure. Oddly, the antibody production and increased clearance of the drug do not decrease the response of the tumor to the treatment. Denileukin is a highly toxic compound, and the more serious toxicities decrease with continued exposure to the agent. The cost of therapy ranges from $14,888-$24,813, depending on the dose regimen used. Also there are additional costs associated with the management of the toxicities caused by denileukin. Potential future uses include: earlier use in the treatment of cutaneous T-cell lymphoma (CTCL), use in CD25-negative patients with CTCL, and use in non-Hodgkins lymphoma.
New Supportive Care Therapies and Management Strategies
Pharmacists have traditionally been involved in the management of patients receiving chemotherapy. Pharmacists are often consulted on the management of chemotherapy-related toxicities such as nausea and vomiting and myelosuppression. Pharmacists need to be involved in managing all issues related to the patient's therapy, including radiation and surgery-related issues. Traditional methods of approaching patient care problems must continually be applied to managing issues for cancer patients; however, it is important to address a problem while also trying to understand the impact of the problem on the patient and the cause of the problem. In evaluating patients one must take the following steps:
- Evaluate the situation.
- Develop a patient-specific plan.
- Implement the plan for management of the problem.
- Re-evaluate the success of the plan.
- Modify the treatment based on the patient's response.
Cancer patients experience many treatment-related problems. Management of the treatment-related problems is complex, as they coexist with numerous other problems.
Treatment-related problems include, but are not limited to:
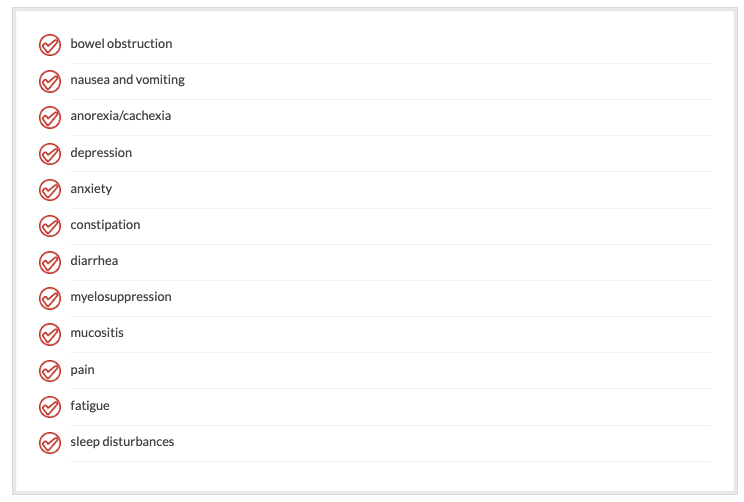
Understanding the pathophysiology of cancer and treatment-related problems has increased our ability to treat these symptoms more effectively.
Managing Cancer Pain
New discoveries in the pathophysiology of cancer pain have led to the development of therapies that may increase our success in treating this problem. Cancer pain is persistent pain, but there are episodes of breakthrough. Standard practice in determining analgesic doses is based on empiric daily analgesic requirements. It has been found that there is a lack of correlation between the dose required for breakthrough pain and the dose determined by evidence-based guidelines. Common practice in the past was to increase the running dose of analgesic to prevent episodes of breakthrough pain. These attempts resulted in increased daily consumption of opiates and also increased side effects from the pain medication.
The N-methyl-D-aspartate (NMDA) receptor plays an important role in the mediation of pain in cancer patients. NMDA does not participate in all pain pathways, but there is evidence that using agents that antagonize NMDA receptors may actually lead to decreased opiate requirements. Dextromethorphan is an NMDA antagonist that has been shown to decrease daily morphine requirements when used in combination with morphine (in a 1:1 ratio) for cancer-related pain. In a practical application, more hospice patients are being treated with the combination of morphine and dextromethorphan in order to provide more consistent pain relief as well as to decrease the potential side effects that commonly arise from over-medication with breakthrough medications for pain. Pharmacists should be aware that patients may require titration of their opiates when they are used in conjunction with dextromethorphan.
Treatment of neuropathic pain has been a complicated issue for practitioners managing cancer patients. Neuropathic pain is caused by peripheral sensitization. Agents shown to have efficacy in the treatment of neuropathic pain include anticonvulsants, capsaicin, and neurokinin-1 receptor antagonists. Opiates are also active in the management of neuropathic pain, despite a common misconception that they are ineffective in treating this type of pain. The anticonvulsants vary in their mechanism of action, and therefore they are not cross-sensitive in their activity. Patients may fail one class of anticonvulsants and respond to another. Gabapentin has had the biggest impact on the treatment of neuropathic pain. There are few clinical trials supporting its use in this setting; however, gabapentin shows significant activity in treating neuropathic pain. In considering the agent of choice when using anticonvulsants for pain management, the pharmacist must keep in mind the toxicities and drug interactions associated with the potential agents.
Bisphosphonates have traditionally been used for the management of hypercalcemia related to certain malignancies. Bisphosphonates also are used in the management and prevention of skeletal complications in patients with metastatic breast cancer, multiple myeloma, and possibly prostate cancer. There is also some evidence supporting the role of bisphosphonates in decreasing bone disease associated with certain malignancies. Bisphosphonates are used routinely in the outpatient setting, and there has been great controversy over infusion rates. There is recent evidence to suggest that there is no difference in efficacy or toxicity between a 1-hour infusion of pamidronate and a 4-hour infusion of pamidronate in patients with multiple myeloma. [3] The newer bisphosphonates under investigation are significantly more potent than those currently on the market. The potency of the bisphosphonates is listed in Table 2.
Table 2. Bisphosphonate Potency
View Table 2. Bisphosphonate Potency
| Drug | Relative Potency | |
| Etidronate | 1 | |
| Clodronate | 10 | First Generation |
| Tilundronate | 10 | |
| Pamidronate | 100 | Second Generation |
| Alendronate | 1000 | |
| Ibandronate | 10,000 | Third Generation |
| Zolendronate | 20,000 |
Alternative methods of drug administration should always be considered for the management of pain in cancer patients. Transmucosal delivery systems (ie, fentanyl lollipops) have an advantage because of the increased surface area in the mouth as well as the controlled pH and temperature. On the other hand, rectal administration of medications should only be considered in those medications approved for that route of administration. Differences in absorption and dissolution may lead to erratic drug delivery and may complicate evaluation of the patient's opiate needs.
Management of Fatigue
Lack of energy is a significant problem in cancer patients. Many cancer patients say that fatigue is the primary symptom that prevents them from leading a normal life. Fatigue decreases productivity in those patients still trying to work through their therapy. There is a definite correlation between hemoglobin concentration and quality of life in cancer patients. Erythropoietin (EPO) is the agent of choice in treating chemotherapy-related anemia and the associated fatigue. Studies have shown that weekly dosing of EPO is equal in efficacy and safety to the 3 times weekly injections originally used in cancer patients.
Management of Insomnia
Lack of energy is a significant problem in cancer patients. Sleep disturbances are the most frequently overlooked problem in cancer patients. Sleep disturbances may be due to depression, pain, anxiety, cognitive impairment, and many other factors. It is important for pharmacists and other health practitioners to evaluate a patient's sleep disorders. It is important to remind the patient about sleep hygiene:
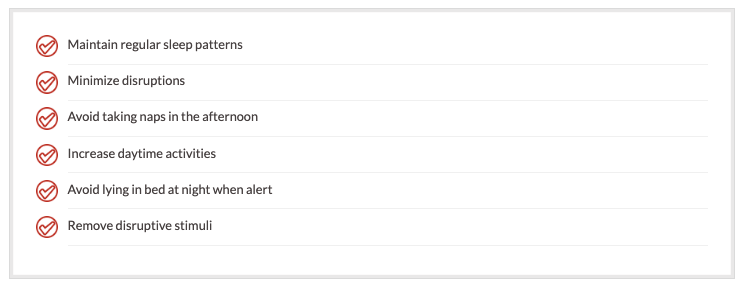
Managing Cancer-related Weight Loss and Anorexia
There are several agents that are effective in managing anorexia and weight loss in cancer patients. Corticosteroids have traditionally been used, and while they are effective in increasing quality of life and stimulating appetite, they are wrought with side effects that make them less than desirable in some patients. Megestrol acetate has been shown to be equally efficacious to dexamethasone in stimulating appetite and preventing weight loss in cancer patients. Dexamethasone is associated with a high incidence of steroid-related toxicities, while megestrol acetate is associated with an increased risk for deep venous thrombosis in cancer patients.
Managing the Febrile Neutropenic Patient
With changes in antibiotic prophylaxis and the use of growth factors following chemotherapy, there has been a shift in the bacterial organisms being treated in cancer patients. In the past, emphasis was placed on preventing gram-negative infections, while current practices are placing more emphasis on the prevention of gram-positive organisms. There is also a trend toward treating febrile neutropenia in the outpatient setting, which makes the role of the pharmacist even more important. There is a great need to have sound guidelines for evaluating patients who are considered low-risk and therefore eligible to be followed in the outpatient setting. This places a large amount of responsibility on the pharmacist to educate the patient about the appropriate use of their antibiotics and proper monitoring for infection. The success of outpatient management of febrile neutropenia is largely dependent on patient selection (expected duration of neutropenia, comorbid diseases, previous treatment with fluoroquinolones, clinical indication for antibiotic therapy).
Patient Education vs Patient Information
Patients have many sources for retrieving information about their illnesses and treatment-related toxicities. The Internet is the largest source of information for the patient. The pharmacist must ensure that the patient not only receives drug information but that the patient or care giver is educated about medication use and expected toxicities. The pharmacist must have some way of measuring comprehension, be able to follow patient compliance, and assist in monitoring for toxicities of therapy. There must be a continuum of care for the patient, and communication about changes in therapy must be documented in order to ensure compliance with the treatment plan. The treatment plan must be communicated clearly to the patient or care giver so they can be involved in monitoring and documenting outcomes.
Cancer Drugs of the Future
Where are we going with cancer drug therapy? The original thought process on designing chemotherapeutic agents focused on the belief that cancer cells are multiplying too quickly, and therefore we should design drugs that destroy rapidly dividing cells. We quickly saw that normal cells were affected as well as the tumor cells. Therefore, cancer therapy is becoming more targeted, and significant discoveries in cancer cell biology have been made, which have led to the development of new theories for attacking cancer cells.
Using Old Drugs Better
Newer agents are being developed to reverse multidrug resistance (MDR), which will allow further development of older drugs in the treatment of previously resistant disease.
New Molecular Targets
Agents are being developed that focus on specific pathways in the cell cycle. These include:
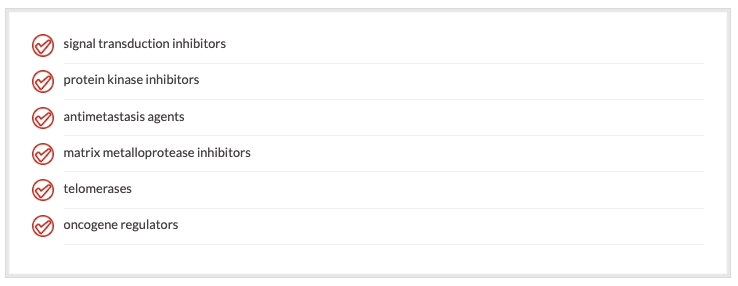
These newer targets allow for the development of specific therapies that are more effective and less toxic to the patient.
Understanding Cancer Biology
Over the past 25 years there have been significant accomplishments in defining the cell cycle. As newer pathways within the cell cycle have been defined, it has led to the development of new targets for therapy and has also explained certain biologic processes that have led to treatment failure.
Drug Resistance
There are several reasons why a cell may be resistant to chemotherapy:

Drugs have been targeted to block the MDR transporter pumps. Early developments used common drugs such as verapamil and cyclosporine A to block the efflux pumps of resistant cells. Success of these agents was limited by toxicity. A new agent, PSC-833 (Valspodar), is close to FDA approval for use in combination regimens for acute myelogenous leukemia (AML). It has been shown that PSC-833 increases the retention of daunorubicin in cells and also increases the cytotoxicity of daunorubicin on the leukemic cells.
P-glycoprotein-mediated MDR
Many drugs are affected by the MDR efflux pumps. Many of them are natural products, and they have different mechanisms of action. It is believed that one of the mediators of MDR is p-glycoprotein. P-glycoprotein is believed to mediate MDR for the following agents:
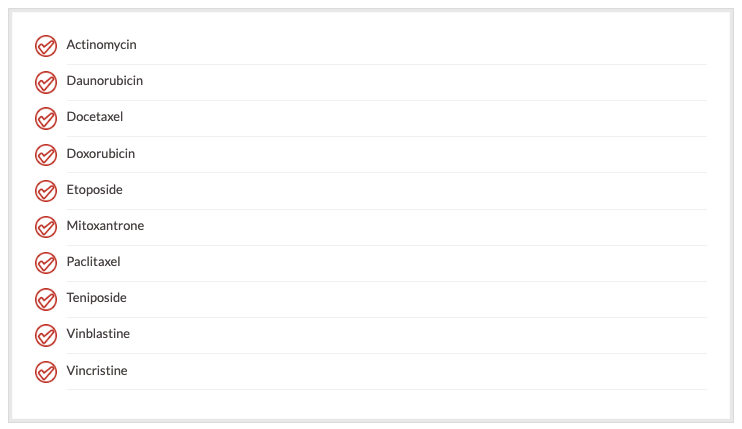
P-glycoprotein is also expressed in many normal tissues, such as brain, small bowel, pancreas, liver, adrenal glands, colon, kidney, and testis. Many of these organs are natural detoxifiers for the body. Blocking the efflux pumps with agents such as PSC-833 may lead to specific toxicities due to the presence of p-glycoprotein in these organs.
Pharmacists should be cautioned that the use of agents to block MDR should be strictly monitored. Agents to block MDR will be approved for use with specific regimens, and dose reductions of the chemotherapeutic agents may be needed to avoid excessive toxicity. Consideration should be given to whether the cytoxicity is due to increased drug activity or increased exposure of the cells to the chemotherapeutic agent.
Signal Transduction
Signal transduction cascades are activated by mitogens, stress, and hormones. The cell receives signals that tell it to continually divide when it should have already gone through cell death. New agents have been designed to block signal transduction. Some of the more promising agents include:
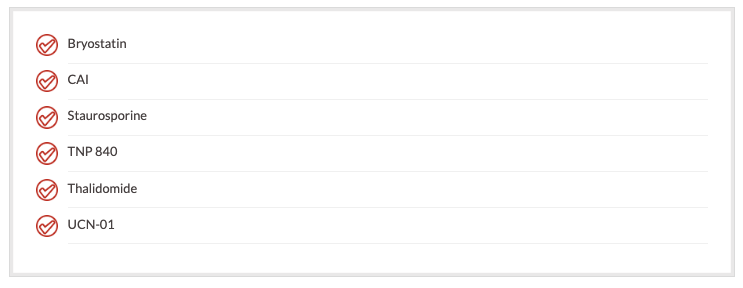
Cyclin-dependent Kinases
Cyclin-dependent kinases interact with molecules and tell the cell to go through the cell cycle process. The cyclin-dependent kinases work in all aspects of the cell cycle. Newer agents under investigation to block the cyclin-dependent kinases are:
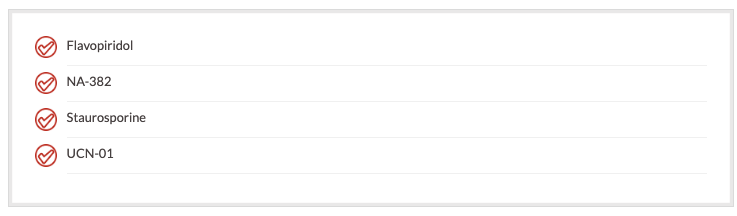
Anti-angiogenisis
Angiogenesis is a process by which a cell induces neovascularization. It is believed that inhibiting angiogenesis may also prevent metastatic disease. Anti-angiogenesis agents under investigation include:
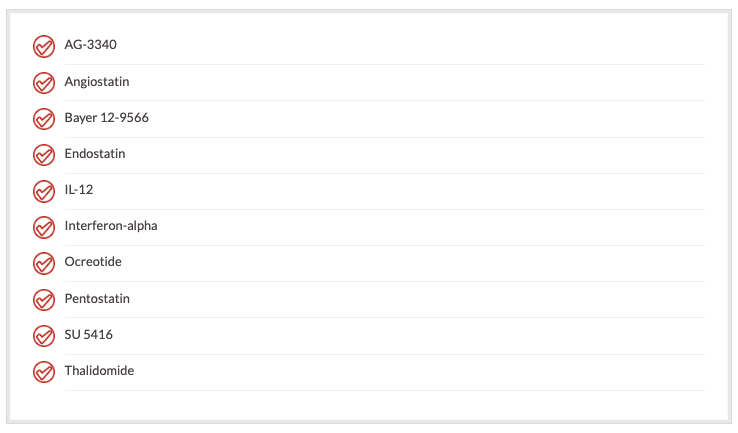
Tumor Suppressor Genes
Tumor suppressor genes exist in many cell lines. p-53 is a gene that has tumor suppressor activity. It has been demonstrated that lack of p-53 in cells is a poor prognostic factor for response to chemotherapy. Onyx-015 is an agent that has been designed to mimic p-53 in cells that are p-53 deficient or have defective p-53. In the cell, Onyx-015 replicates through a viral vector and causes cell death. The viral vector then can be transmitted to other cells.
Apoptosis is "programmed cell death." Many developments have been made to determine the events leading up to the terminal event for a cell.
Cancer Vaccines
Cancer vaccines are a highly specific targeted approach to cancer therapy. The biggest barrier to cancer vaccine therapy is trying to find a way to present the antigen to the immune system to activate the immune system against the tumor cells. Methods have been used to load the antigens onto presenting cells (such as fibroblasts), which allow the immune system to be activated. Adjuvant agents have been used (such as interferon-gamma) to enhance T-cell response to the antigen and increase the retention of the vaccine within the cell. So far, vaccine therapy has been used in melanoma, colon cancer, and prostate cancer.
Targeted Toxins
Targeted toxins are another specific method to deliver cytotoxic agents to a cell. There are 2 components--the targeting domain (needs a specific receptor site in the cell) and the toxic domain (a diphtheria, ricin, or Pseudomonas component). The toxin is internalized and inhibits protein synthesis at a ribosomal level. The limitation is that the cells must express the target receptor, and development of these compounds is labor intensive and expensive. One new cancer agent that was designed as a targeted toxin is denileukin deftitox.
Gene Therapy
Gene therapy is the most patient-specific targeted approach to treating cancer. Strategies such as using lymphocytes as the gene carrier and gene-based immunotherapy have not proven to be effective methods of gene manipulation for cancer treatment. More promising data in gene therapy have come from direct injection of genes, which allows insertion of missing immune recognition antigens as well as the introduction of sensitivity genes. There are also therapies targeted at replacing missing or mutant tumor suppressor genes.
Manipulation of the gene must include methods that will increase the immune stimulation in response to the gene. Incorporation into a liposome is a practical approach to delivering a gene into the patient. Sensitivity genes are virally directed and take advantage of the transcriptional differences between normal cells and tumor cells. Insertion of the gene not only causes a "suicide" effect to the cell, but also creates a bystander effect that results in selective destruction of transduced cells. Through gene therapy, you can replace a missing or mutant tumor suppressor gene and force that cell into a normal phenotype.
Future Directions of Cancer Therapy
With the identification of gene sequencing and the progress made by the Human Genome Project, the future of cancer therapy will be focused at the chromosomal level. Therapies will be targeted at preventing disease, identifying genetic abnormalities, and developing agents that will trick tumor cells into self-destruction without causing any toxicity for the patient.

Our Mission
-
Medical Empowerment
Get the information you need to make informed decisions about your treatment.
-
Legal Empowerment
Get the compensation you need to address the financial cost of your illness.
-
Going For The Gold
Helping empower our clients with over $2.5 Billion in recoveries. We fight for our clients!
-
Giving Back To The Community
Unprecedented Support for Mesothelioma Research. See how we can help!



